
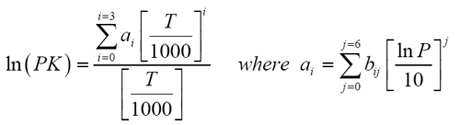
The rest is withdrawn as bottoms, or bottoms product Liquid at the bottom of the column is partially vaporized in a heated reboiler. Liquid runs down the column from tray to tray, where as vapour is ascending along the column.Īt each tray vapour and liquid contact and mix with each other The feed is material is introduced at one or more points along the column.

La, xa=xd=y1=ya condenser Ln-1 xn-1 Vn yn This process takes advantage of the differences in distribution of components between the vapour and liquid phase. Relative volatility, αAB If AB is independent of T, the equi composition can be calculated byĭistillation is a process where a feed mixture of two or more components is separated into products, of compositions different from the feed. Relative volatility, The relationship between the composition of the vapor yA and the liquid xA in equi can also be expressed calls volatility. PA = PAo xA For an ideal mixture, the partial pressure is related to the concn in a liq. P = PA PA = yA P In an ideal gas/vapor, the partial press is ≈ to the mol fraction Partial pressure, Dalton’s & Raoult’s Law Dalton’s Law In separations, we observe a process from the macroscopic point of view. Micros scale Condensing and evaporating continue but the rates of them are the same. Macros scale No further changes can be observed in T, P & compositions. Phase equi the rate of changing from one phase to another is = to the rate of the reverse change. In other words, streams leaving a separation stage are in equilibrium.Ī state of balance between opposing forces or actions that is either static or dynamic A state of intellectual or emotional balanceĬhemical equi rates of reaction in both directions are =. The two separated phases are assumed to be in EQUILIBRIUM with each other.
Depriester chart bubble point series#
The results are conveniently shown on a Temp-composition diagram as shown:Įquilibrium Staged Separation…? A separation process can be constructed as a series of discrete stages in which the two phases are contacted and separated. The composition of the vapor in equilibrium with a liquid of given composition can be determined experimentally using an equilibrium still.


 0 kommentar(er)
0 kommentar(er)
